Many diseases are “multimechanistic”.
Multimechanistic conditions involve more than one biological pathway. Accordingly, they require more than one treatment approach. The different mechanisms underlying symptoms can be shared by more than one disease or condition.
As an example, the etiology of pain can be neurological or inflammatory, and the mechanisms involved can be modulated centrally or peripherally. Most therapeutics target a single receptor or pathway, leaving gaps in treatment. BRC’s therapies are designed to address multiple mechanisms both within and beyond the nervous system, offering a more comprehensive approach to care.
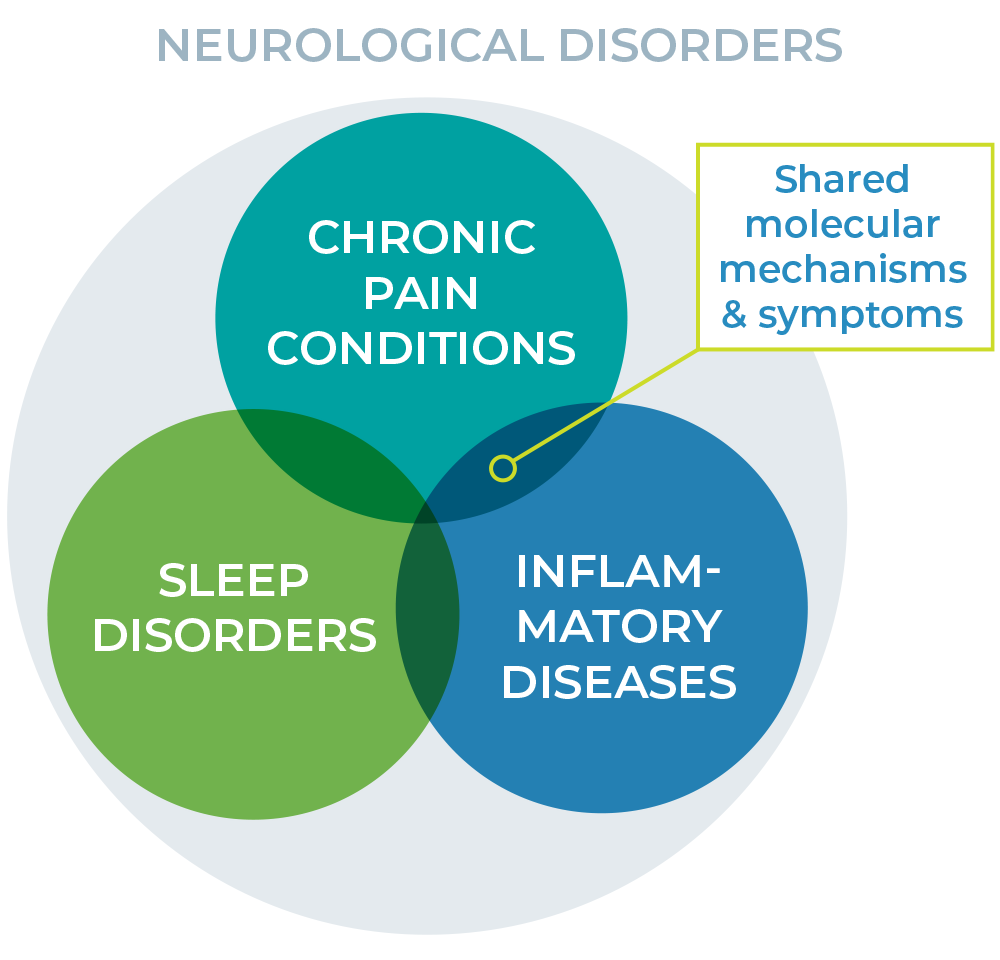
Within neurological conditions, overlapping molecular mechanisms contribute to shared symptoms and treatment challenges.
Multimodal therapies meet the needs of patients.
Multimodal therapeutics are uniquely suited to address multimechanistic diseases and conditions. They affect more than one mechanism and thus better meet the needs of individual patients. Traditional multimodal therapeutics are combinations of medicines in standardized ratios, such as fixed-dose combination medicines. BRC’s multimodal therapies are designed as a single treatment.
Cannabinoids are multimodal medicines addressing serious diseases.
BRC Therapeutics is working to develop treatments that harness the unique benefits of multiple cannabinoids with different mechanisms of action, all in a single prescription medicine. The multimodality of cannabinoids is rooted in their divergent modulation of receptors, ion channels, and associated signaling cascades. The interaction of multiple cannabinoids at a single receptor can influence their combined activity.
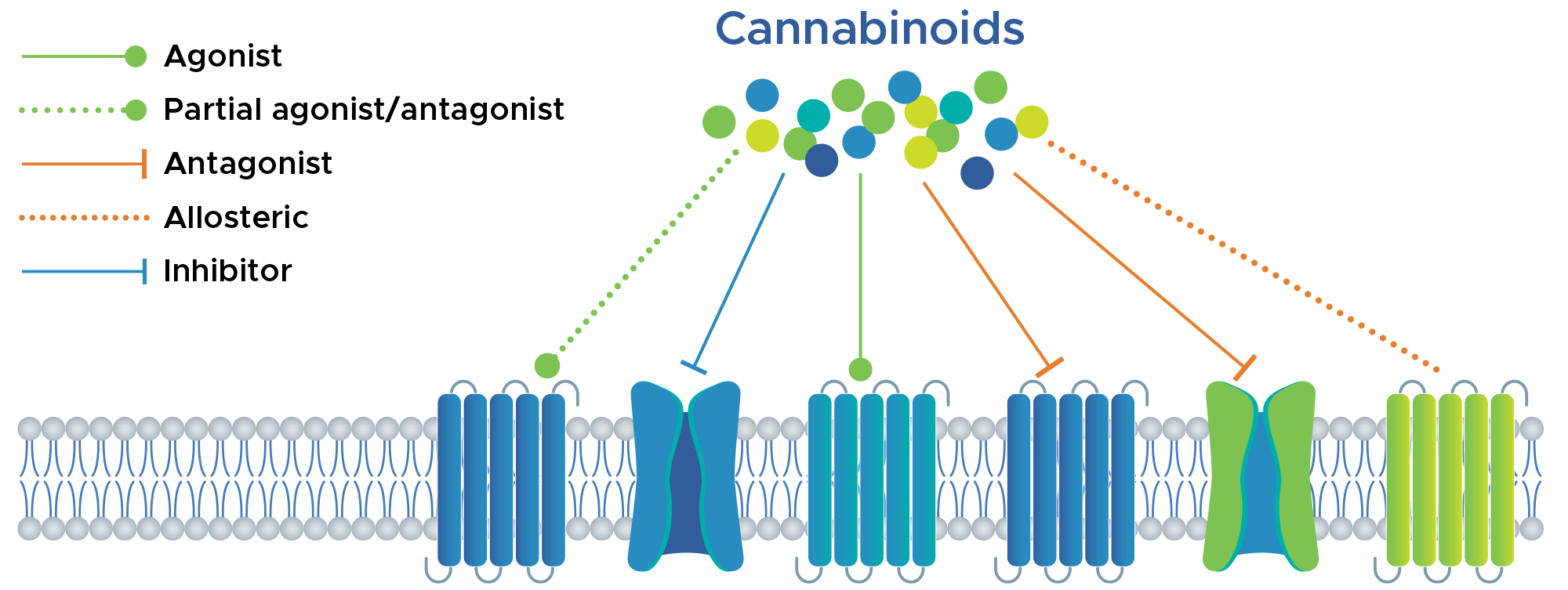
Proposed multimodal actions of cannabinoids
Tailoring medicines to disease complexity and to individual requirements.
BRC’s multimodal therapeutic approach is expected to maximize efficacy of combinatorial treatments while minimizing adverse effects. This innovative strategy enables effective treatment of complex diseases driven by multiple mechanisms, offering patients a more comprehensive solution currently unavailable with conventional therapies.
BRC Therapeutics has a strong IP portfolio covering cannabinoid compositions, including key cannabinoid components in the drug substance and drug product.
Our portfolio also covers the use of BRC’s proprietary cannabinoid compositions in treating various indications, including inflammatory diseases, neurological conditions, and pain.
BRC Therapeutics has been awarded the appropriate controlled substances registrations for all research, development, manufacturing and commercial activities by the Drug Enforcement Administration (DEA). Federal compliance for botanically derived cannabinoids across the value chain enables drug development both in-house and with partners, and intellectual property generation.
Complete portfolio of DEA registrations
| Schedule 1 Analytical | Awarded 2019, Active |
| Schedule 1 Distribution | Awarded 2020, Active |
| Schedule 1 Manufacturer (Cultivation and Manufacturing) |
Awarded 2021, Active |
| Schedule 1 Importer | Awarded 2021, Active |
| Schedule 1 Exporter | Awarded 2021, Active |

