Following a rigorous manufacturing process.
BRC’s medicines are botanically derived and contain cannabinoids at defined ratios. In 2020, we completed the implementation of a quality system for all stages of production, including primary processing. We have been operating under cGMP since 2023.
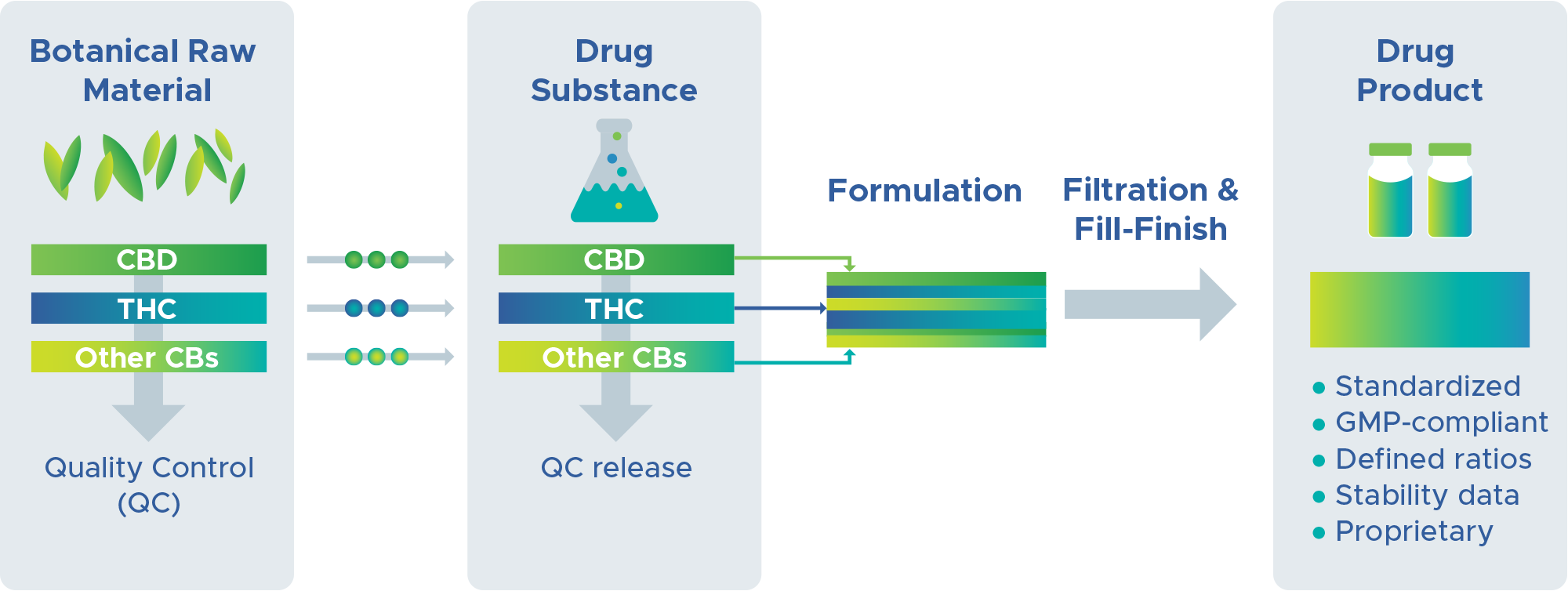
Our processes transform botanical sources into standardized medicines.
BRC Therapeutics operates a fully equipped facility for growing plants and processing botanical raw materials. For starting materials, we strictly adhere to GACP, and we document all conditions during cultivation, collection, and harvest. We follow standardized GMP-compliant procedures for extraction and formulation of drug substances at defined ratios towards generation of the finished drug product. Our first GMP release of drug substances and product occurred in Q4 2023.